Graphical Abstract
This updated British Society for Haematology guideline provides an up-to-date literature review and recommendations regarding the identification and management of preoperative anaemia. This includes guidance on thresholds for the diagnosis of anaemia and the diagnosis and management of iron deficiency in the preoperative context. Guidance on the appropriate use of erythropoiesis-stimulating agents and preoperative transfusion is also provided.
The previous version of this guideline was published in 20151 and provided a comprehensive review of the available literature. Since then, a number of key clinical trials have reported findings relevant to the investigation and management of preoperative anaemia including those considering optimal dosing of oral iron2, 3 and the use of intravenous iron for patients undergoing major surgery.4, 5
The SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) pandemic has placed extra importance on the appropriate management of preoperative patients, with a now huge number of patients6 awaiting elective surgical procedures postponed by the pandemic. All UK nations have published recovery plans, which outline actions that will be undertaken to address the care backlog, including those affecting elective surgery.7-10 A BSH Good Practice Paper published in 202111 made recommendations for best patient blood management (PBM) while working within the limitations imposed by the need to minimise hospital attendances to reduce the risk of SARS-CoV-2 infection. Many of the recommendations made at that time remain relevant and where appropriate have been incorporated into the current guideline.
While blood stocks are currently good, the blood supply has been challenging, with NHS Blood and Transplant declaring an Amber alert in October 2022.12 This unprecedented event led to a rapid push to implement PBM wherever possible to reduce demand for blood. Managing the demand for blood in elective surgical patients by appropriately identifying and managing anaemia in the preoperative setting is therefore important not only at an individual patient level but also at a system-wide level to ensure blood is available for all who need it.
This updated guideline is cognisant of the role of Primary Care in the management of these patients, and this is reflected in the writing group membership as well as the recommendations made. The guideline writing group is pleased to have representation from all four UK nations to ensure this guidance is widely applicable. It sits alongside the recently published Centre for Perioperative Care guideline for the management of anaemia in the perioperative pathway.13 This BSH guideline provides an up-to-date literature review with recommendations. Implementation should be informed by local circumstances and integrated into exiting care pathways.
The association of preoperative anaemia with patient outcome after surgery
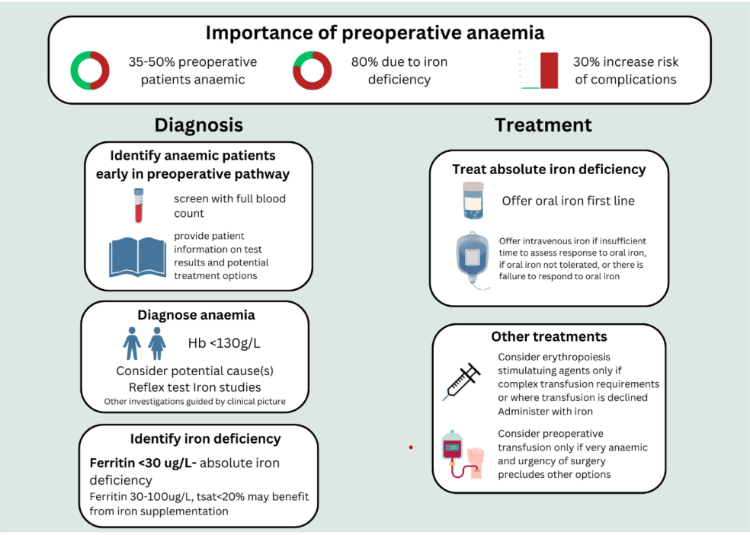
Anaemia is associated with an increased likelihood of requiring transfusion, as well as mortality and morbidity after major surgery. Preoperative anaemia is also, in principle, modifiable with appropriate treatment though it is unclear under what circumstances such modification translates to patient benefit. Definitions of PBM have evolved over the years since its inception, with the recent publication of a global definition as follows: ‘PBM is a patient-centred, systematic, evidence-based approach to improve patient outcomes by managing and preserving a patient's own blood, while promoting patient safety and empowerment’.14 Preoperative anaemia diagnosis and management therefore falls under the PBM umbrella. Patients requiring surgical intervention who present with anaemia are more likely to receive a transfusion of packed red cells. Preoperative anaemia and blood transfusion have been associated with increased morbidity and mortality as shown by several systematic reviews and observational studies.15-17 Studies reviewing the prevalence of anaemia in the preoperative population vary but demonstrate up to 35%–50% of preoperative patients to be anaemic,18-20 this being influenced by the presenting disease, that is, cancer population, the burden of comorbidities, age, gender and surgical procedure.
The severity of anaemia has also been linked to outcomes.16 Patients with a haemoglobin (Hb) concentration >100 g/L, classified as mildly anaemic, had a 30% increased relative risk of complications and death.
The identification of links between preoperative anaemia and adverse outcomes has led to further investigation by perioperative care teams into the causes of anaemia in the surgical patient. Hung et al. demonstrated by bone marrow sampling of anaemic patients presenting for cardiac surgery that most (80%) were iron deficient.21 This figure was corroborated in patients undergoing major abdominal surgery in the PREVENTT study where 82% had iron deficiency.22 Recent perioperative guidelines suggest identifying and managing the underlying cause of anaemia is essential in its management.13 Transfusing a patient for haematinic deficiencies without a significant symptom burden to justify it exposes patients to unnecessary risks associated with transfusion such as transfusion reactions, fluid overload, incorrect component transfusions and, more rarely, infection.23 Transfusion for haematinic deficiency in the absence of symptoms is therefore reportable to Serious Hazards of Transfusion (SHOT) as an avoidable transfusion.24 Transfusing without correcting the haematinic deficiency also leaves the patient at risk of the adverse effects of the deficiency itself and a relapse of the anaemia as the transfused red cells senesce.
- Assessment for anaemia in patients undergoing elective surgery should be performed early in the preoperative pathway (1C).
- Patients undergoing major surgery should be screened for anaemia by full blood count (including red cell indices) in the first instance (2B).
- Patients should be provided with information regarding the results of preoperative screening tests and potential treatment options to allow for shared decision-making regarding further management (2B).
Declaration of Interests
The BSH paid the expenses incurred during the writing of this guidance. All authors have made a declaration of interests to the BSH and Task Force Chairs which may be viewed on request. JD has worked as a consultant for the WHO Departments of Nutrition and Food Safety and the Maternal Health Unit within the Department for Maternal, Newborn, Child and Adolescent Health and Ageing. This has included personal fees for work on postpartum anaemia, multiple micronutrients and intravenous iron use in women of childbearing age. CE has received educational grants and honoraria for speaker roles from Pharmacosmos. AK has received consultancy fees from Pharmacosmos and speaker fees from Vifor Pharma. AW has received honoraria for presentations from Takeda, AbbVie and Pharmacosmos. ST received consultancy fees from Pharmacosmos for support of an experienced practice group for the management of IDA. TR has received departmental reimbursement from BioAge Labs and Viatris. The following members of the writing group: KH, SN and CT have no conflicts of interest to declare.

